About Cellenkos
We are a patient-centric, clinical stage, allogeneic T regulatory cell therapy biotechnology team focused on treating Rare Inflammatory Diseases.
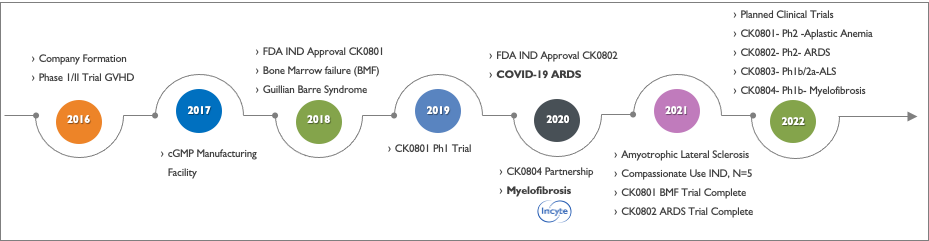
Cellenkos was founded in 2016 with the licensing of a proprietary umbilical cord blood T-Regulatory (Tregs) cell therapy platform from MD Anderson Cancer Center and funded by Golden Meditech Holdings Ltd, a Hong Kong-based integrated healthcare enterprise.
Because they are derived from umbilical cord blood, Cellenkos’ Tregs are naïve, bonafide suppressor cells that resolve inflammation through multiple direct and indirect interactions.
Cellenkos is dedicated to the development and commercialization of allogeneic, off-the-shelf cell based products for the treatment of various inflammatory diseases and autoimmune disorders. Cellenkos’ Tregs have the potential to treat a diverse array of medical issues.
Management Team
Scientific Advisory Board
Investors and Partners
Research Partner
The University of Texas MD Anderson Cancer Center in Houston ranks as one of the world's most respected centers focused on cancer patient care, research, education and prevention. It is one of only 41 comprehensive cancer centers designated by the National Cancer Institute (NCI). For the past 25 years, MD Anderson has ranked as one of the nation's top two cancer centers in U.S. News & World Report's annual "Best Hospitals" survey. It receives a cancer center support grant from the NCI of the National Institutes of Health (P30 CA016672).
MD Anderson Cancer Center and Cellenkos collaboration will support the T-REG Program.
ASD: AUTISM SPECTRUM DISORDER
AD: ALZHEIMER’S DISEASE
MS: MULTIPLE SCLEROSIS
IBD: INFLAMMATORY BOWEL DISEASE
GVDH: GRAFT VS HOST DISEASE
AA: APLASTIC ANEMIA
SLE: SYSTEMIC LUPUS ERYTHEMATOSIS
RA: RHEUMATOID ARTHRITIS
T1D: TYPE 1 DIABETES
Please click here to review institutional conflict of interest management plan.